
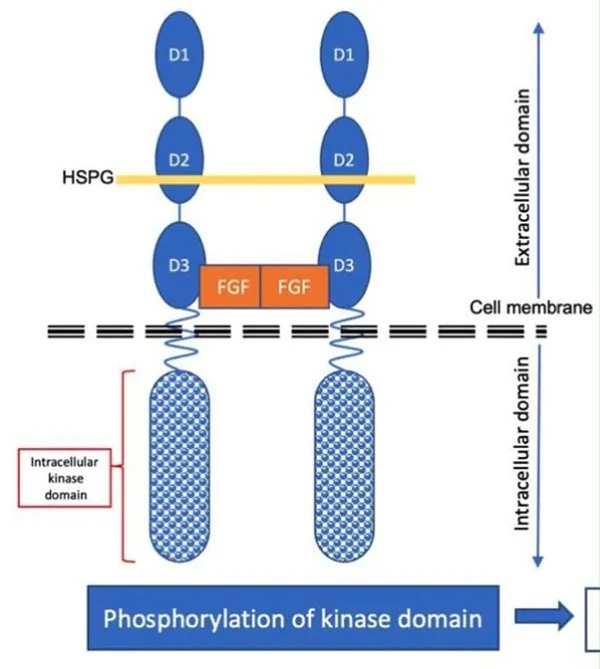
FGFR2b是一种重要的信号传导受 体,涉及多个细胞过程,包括细胞增殖、分化、迁移和存活。FGFR2b信号通路的详细介绍如下:
1) FGFR2b激活机制:
Ligand Binding:FGFR2b的活化通常是由FGF(成纤维细胞生长因子)家族的成员诱导的。这类成员包括FGF1、FGF2、FGF7和FGF10等。这些成分通过结合到FGFR2b的细胞外结构域,引发受体的二聚化和自磷酸化,从而启动信号传导。
Receptor Dimerization: 配体结合导致FGFR2b 的二聚化,即两个受体结合在一起形成二聚体。这一过程是信号传导的关键步骤,为后续的下游信号传导做好准备。
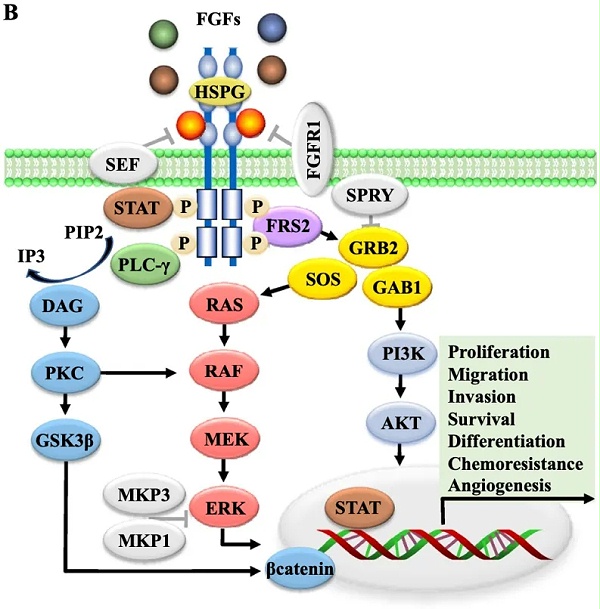
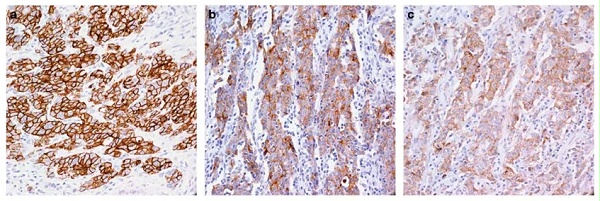
图3:FGFR2b在胃癌中表达
如在乳腺癌中,64 个BRCA2 相 关的肿瘤中有 19 个( 30% )对 FGFR2 呈阳性染色,而 50 个 BRCA1 相关的肿瘤中有 3 个( 6% )对 FGFR2 呈阳性染色( P = 0.004 )。 因此可以得出结论, FGFR2b 的表达更容易在 BRCA2 相关的乳腺癌中上调。
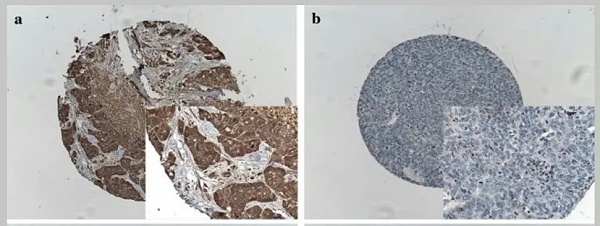
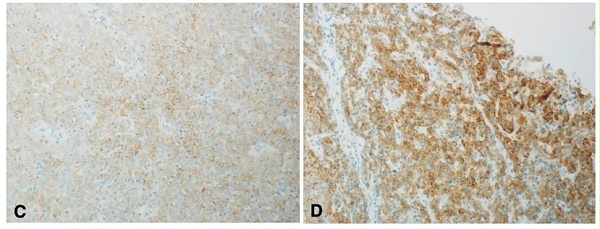
图5:FGFR2b在胆管癌中表达
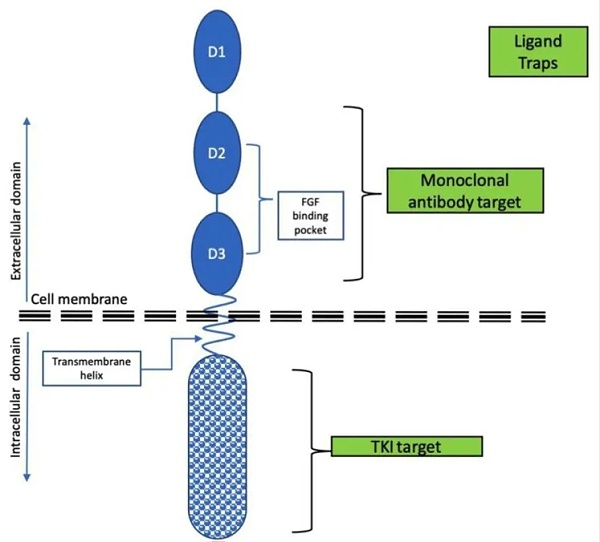
FGFR2靶向药物根据作用机制可分为四类:(1)小分子酪氨酸激酶抑制剂(TKIs),包括非选择性和选择性的FGFR抑制剂;(2)对抗性单克隆抗体,竞争性结合到FGFR细胞外区域,阻止FGFs–FGFR信号的激活;(3)FGF配体陷阱,阻止多种FGF配体的活性和配体-受体相互作用;以及(4)抗体-药物结合物(ADCs),由FGFR抗体与细胞毒性荷载组成。部分药物信息及研发进展见表1。
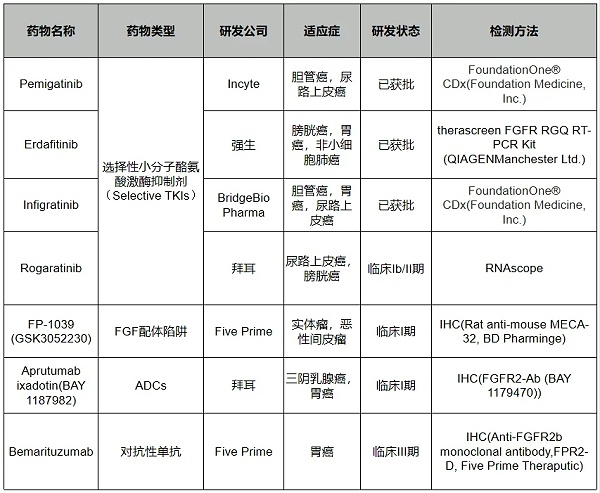
表1:FGFR2b靶向药物信息
FGFR2b的过表达已在包括乳腺、子宫内膜、宫颈、肺、食管、胃、胰腺和结直肠癌 (CRC )的多种癌症中报道,但 F GFR-2 IIIb 变体在不同癌症类型之间的作用存在争议。 在食管癌患者中, 41% 的患者的食管癌细胞中表达了 FGFR-2 IIIb ,并且 FGFR-2 IIIb 的表达与食管癌的良性细胞类型相关。 使用 免疫组织化学( IHC )检测,发现 73 例( 1,974 例中的 4% )胃癌患者中 FGFR2-IIIb 蛋白过表达,并且所有具有 FGFR2-IIIb 过表达的肿瘤均通过荧光原位杂交检测到 FGFR2 扩增。 并且, FGFR2b 在胃癌中的表达尤为显著,在一项研究中,一种新型的 FGFR2b 初级抗体通过免疫组织化学来预测基因扩增,并发现 4% 的胃癌中存在 FGFR2b 过表达,其中 92% 的病例通过 FISH 确认为 FGFR2 基因扩增,证实这是 FGFR2 扩增的胃癌中主要表达的是 FGFR2b 亚型,而不是 FGFR2c 亚型。 值得关注的是,随着 FGFR2b 表达水平的增加,观察到更大的生存获益,说明FGFR2b 靶点或可作为临床中肿瘤疗效预测的指标。
目前,迈杰转化医学研究(苏州)有限公司 已成功开发一株FGFR2b 特异性单克隆抗体,该抗体已证实可用于 FFPE 组 织样本 的 IHC 染色,在胃癌和卵巢癌等组织上有良好的染色性能。 迈杰医学 FGFR2b 抗体试剂( 免 疫组织化学法) 已完成技术可行性研究。 该产品既可满足临床样本检测需要,也可作为 FGFR2b 靶向药物的伴随诊断试剂产品原型。
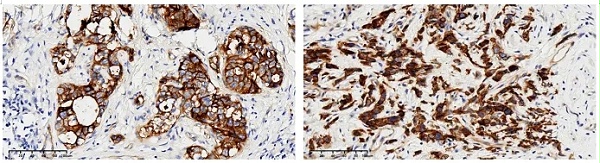
图7 :迈杰医学FGFR2b抗体在阳性胃癌组织上的染色
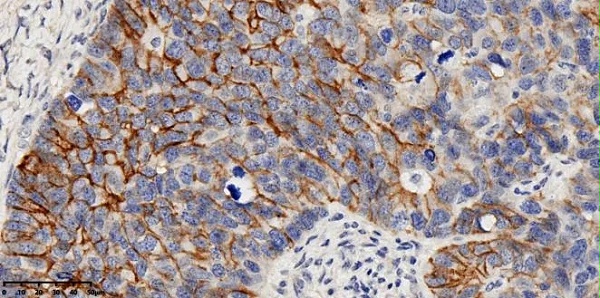
图8:迈杰医学FGFR2b抗体在阳性卵巢癌组织上的染色
[1] Ahn, S., Lee, J., Hong, M., Kim, S. T., Park, S. H., Choi, M. G., Lee, J.-H., Sohn, T. S., Bae, J. M., Kim, S., Jung, S.-H., Kang, W. K., & Kim, K.-M. (2016). FGFR2 in gastric cancer: protein overexpression predicts gene amplification and high H-index predicts poor survival. Modern Pathology, 29(9), 1095–1103.
[2] Ahn, S., Lee, J., Hong, M., Kim, S. T., Park, S. H., Choi, M. G., Lee, J.-H., Sohn, T. S., Bae, J. M., Kim, S., Jung, S.-H., Kang, W. K., & Kim, K.-M. (2016). FGFR2 in gastric cancer: protein overexpression predicts gene amplification and high H-index predicts poor survival. Modern Pathology, 29(9), 1095–1103.
[3] Bane, A. L., Pinnaduwage, D., Colby, S., Bull, S. B., O’Malley, F. P., & Andrulis, I. L. (2008). Expression profiling of familial breast cancers demonstrates higher expression of FGFR2 in BRCA2-associated tumors. Breast Cancer Research and Treatment, 117(1), 183–191.
[4] Blackwell, C., Sherk, C., Fricko, M., Ganji, G., Barnette, M., Hoang, B., Tunstead, J., Skedzielewski, T., Alsaid, H., Jucker, B. M., Minthorn, E., Kumar, R., & DeYoung, M. P. (2016). Inhibition of FGF/FGFR autocrine signaling in mesothelioma with the FGF ligand trap, FP-1039/GSK3052230. Oncotarget, 7(26), 39861–39871.
[5] Choi, C. H., Chung, J.-Y., Kim, J.-H., Kim, B.-G., & Hewitt, S. M. (2016). Expression of fibroblast growth factor receptor family members is associated with prognosis in early stage cervical cancer patients. Journal of Translational Medicine, 14(1).
[6] Daniel V.T. Catenacci, Tesfaye, A., Tejani, M., Cheung, E., Eisenberg, P. D., Scott, A. J., Eng, C., Hnatyszyn, J., Marina, N., Powers, J., & Wainberg, Z. A. (2019). Bemarituzumab with modified FOLFOX6 for advanced FGFR2-positive gastroesophageal cancer: FIGHT Phase III study design. Future Oncology, 15(18), 2073–2082.
[7] Gordon, A., Johnston, E., Lau, D. K., & Starling, N. (2022). Targeting FGFR2 Positive Gastroesophageal Cancer: Current and Clinical Developments. OncoTargets and Therapy, 15, 1183–1196.
[8] Grünewald, S., Politz, O., Bender, S., Héroult, M., Lustig, K., Thuss, U., Kneip, C., Kopitz, C., Zopf, D., Collin, M., Boemer, U., Ince, S., Ellinghaus, P., Mumberg, D., Hess‐Stumpp, H., & Ziegelbauer, K. (2019). Rogaratinib: A potent and selective pan‐FGFR inhibitor with broad antitumor activity in FGFR‐overexpressing preclinical cancer models. International Journal of Cancer, 145(5), 1346–1357.
[9] Hu, M., & Zhang, S. (2022). Expression and clinical significance of FGFR1 and FGFR2 in laryngeal squamous cell carcinoma. Translational Cancer Research, 11(9), 3222–3234.
[10] Ishiwata, T. (2018). Role of fibroblast growth factor receptor-2 splicing in normal and cancer cells. Frontiers in Bioscience, 23(2), 626–639.
[11] Javle, M., Roychowdhury, S., Kelley, R. K., Sadeghi, S., Macarulla, T., Weiss, K. H., Waldschmidt, D.-T., Goyal, L., Borbath, I., El-Khoueiry, A., Borad, M. J., Yong, W. P., Philip, P. A., Bitzer, M., Tanasanvimon, S., Li, A., Pande, A., Soifer, H. S., Shepherd, S. P., & Moran, S. (2021).Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. The Lancet Gastroenterology & Hepatology, 6(10), 803–815.
[12] Masakazu Yashiro, Kuroda, K., Masuda, G., Okuno, T., Miki, Y., Yamamoto, Y., Sera, T., Sugimoto, A., Komatsu, S., Nishimura, S., Shingo Togano, & Ohira, M. (2021). Clinical difference between fibroblast growth factor receptor 2 subclass, type IIIb and type IIIc, in gastric cancer. Scientific Reports, 11(1).
[13] Ooki, A., & Yamaguchi, K. (2021). The beginning of the era of precision medicine for gastric cancer with fibroblast growth factor receptor 2 aberration. Gastric Cancer, 24(6), 1169–1183.
[14] Tolcher, A. W., Papadopoulos, K. P., Patnaik, A., Wilson, K., Thayer, S., Zanghi, J., Gemo, A. T., Kavanaugh, W. M., Keer, H. N., & LoRusso, P. M. (2016). A phase I, first in human study of FP-1039 (GSK3052230), a novel FGF ligand trap, in patients with advanced solid tumors. Annals of Oncology, 27(3), 526–532.
[15] Wainberg, Z. A., Enzinger, P. C., Kang, Y.-K., Qin, S., Yamaguchi, K., Kim, I.-H., Saeed, A., Oh, S. C., Li, J., Turk, H. M., Teixeira, A., Borg, C., Hitre, E., Udrea, A. A., Cardellino, G. G., Sanchez, R. G., Collins, H., Mitra, S., Yang, Y., & Catenacci, D. V. T. (2022). Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): a randomised, double-blind, placebo-controlled, phase 2 study. The Lancet Oncology, 23(11), 1430–1440.